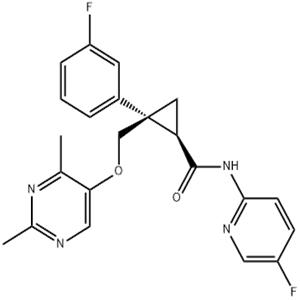
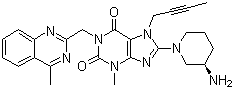
Letermovir is a novel antiviral drug developed by Merck & Co. for the treatment of cytomegalovirus (CMV) infection in CMV-seropositive adult patients who have undergone allogeneic hematopoietic stem cell transplantation (HSCT). It was approved by the U.S. FDA on November 9, 2017, and is marketed under the brand name Prevymis.
Biological Activity
Letermovir (also known as AIC246 or MK-8228) is a novel anti-CMV compound that targets the viral terminase complex. It remains active against CMV strains resistant to DNA polymerase inhibitors.
In Vitro Studies
AIC246 inhibits viral replication without blocking the synthesis of progeny CMV DNA or viral proteins. It is currently one of the most potent agents against human cytomegalovirus (HCMV), with an EC₅₀ of approximately 5 nM in cell-based assays and a selectivity index greater than 15,000.
In Vivo Studies
In mouse xenograft models, mice treated with AIC246 showed dose-dependent reductions in HCMV titers within transplanted cells compared to placebo-treated controls.