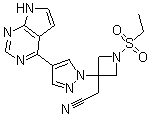
Baricitinib
Product Name: Baricitinib
CAS No.: 1187594-09-7
Purity: ≥99.0%
Category: API (Active Pharmaceutical Ingredient)
Shelf Life: 1 year
Storage: Room temperature, protect from light
Molecular Formula: C16H17N7O2S
Molecular Weight: 371.42
Indication:
Baricitinib is used for the treatment of moderate to severe rheumatoid arthritis (RA).
Mechanism of Action:
A selective JAK1 and JAK2 inhibitor, with IC50 values of 5.9 nM and 5.7 nM respectively. It shows ~70-fold and ~10-fold selectivity over JAK3 and Tyk2, with no inhibitory activity on c-Met or Chk2.
Approval Status:
Originally developed by Incyte and co-developed with Eli Lilly.
-
EMA approved (Feb 14, 2017) under the brand name Olumiant.
-
NDA submitted to FDA in January 2016.
Keywords: 1187594-09-7; Baricitinib; Olumiant; JAK inhibitor; rheumatoid arthritis treatment

![Benzoicacid,4-[5-(3,5-dichloro-4-fluorophenyl)-4,5-dihydro-5-(trifluoromethyl)-3-isoxazolyl]-2-methyl-](https://yikanghuagong.com/wp-content/uploads/2025/07/202311211120417793324-49.jpg)