- Home
- Active Pharmaceutical Ingredient
- Linagliptin
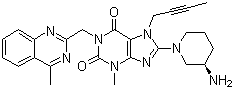
Linagliptin
Product Information
-
Chinese Name: 利拉利汀
-
English Name: Linagliptin
-
CAS Number: 668270-12-0
-
Validity: One year
-
Storage Conditions: Store at room temperature, protected from light
-
Purity: 99.0%
-
Product Category: Active Pharmaceutical Ingredient (API)
Product Description
Linagliptin, developed by Boehringer Ingelheim, was approved by the FDA on May 2, 2011. It is used in combination with diet and exercise to improve glycemic control in patients with type 2 diabetes mellitus. Linagliptin works by inhibiting dipeptidyl peptidase-4 (DPP-4), thereby improving glycemic control in patients.
Compared with other DPP-4 inhibitors, Linagliptin offers the following main advantages:
-
Excellent renal safety profile
-
Effectively reduces glycated hemoglobin (HbA1c) levels
-
Primarily excreted unchanged in feces; only about 5% of the oral dose is excreted via the kidneys
-
Even with intravenous administration, only 30.8% is renally excreted
-
No need for regular liver or kidney function monitoring or dose adjustment
-
Fixed dosing for all patients, making prescription more convenient
Keywords
668270-12-0; Linagliptin; DPP-4 inhibitor; Type 2 diabetes treatment; high purity API; Boehringer Ingelheim; antidiabetic drug; no dose adjustment; renal safety; fixed dose therapy