- Home
- Active Pharmaceutical Ingredient
- Lenvatinib Mesylate
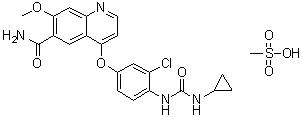
Lenvatinib Mesylate
Product Name: Lenvatinib Mesylate
CAS No.: 857890-39-2
Purity: 99.0%
Category: API
Synonyms:
-
Lenvatinib mesylate
-
E7080 mesylate
-
4-[3-Chloro-4-[[(cyclopropylamino)carbonyl]amino]phenoxy]-7-methoxy-6-quinolinecarboxamide monomethanesulfonate
Molecular Formula: C₂₁H₁₉ClN₄O₄·CH₄O₃S
Molecular Weight: 522.96
Product Description:
Lenvatinib Mesylate is an oral molecular targeted anti-cancer agent. It was approved by the U.S. FDA on February 13, 2015, for the treatment of progressive, locally advanced or metastatic differentiated thyroid cancer (DTC) refractory to radioactive iodine (RAI). It was granted accelerated assessment by the European Medicines Agency, receiving approval just 9 months after submission.
This decision was based on the SELECT Phase III multicenter, randomized, double-blind, placebo-controlled trial. Lenvatinib significantly improved progression-free survival (PFS) compared to placebo.
The study highlighted Lenvatinib’s rapid response, with a median time to first objective response of 2 months. It also demonstrated a statistically significant increase in objective response rate (ORR), including complete responses in 1.5% of patients.
Mechanistically, Lenvatinib selectively inhibits several receptor tyrosine kinases involved in tumor angiogenesis and growth, including VEGFR, FGFR, RET, KIT, and PDGFR. Structural studies reveal that it binds to VEGFR2 in a unique Type II binding mode, enabling potent and rapid inhibition of kinase activity.
Keywords:
857890-39-2; 417716-92-8; Lenvatinib; Lenvatinib Mesylate; E7080