Product Information
-
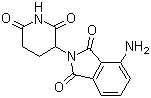
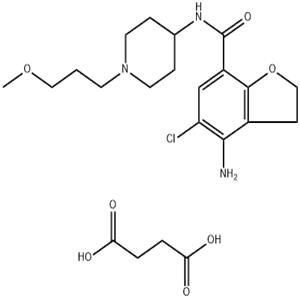
Chinese Name: 泊马度胺
-
English Name: Pomalidomide
-
CAS Number: 19171-19-8
-
Validity: One year
-
Storage Conditions: Store at room temperature, protected from light
-
Purity: 99.0%
-
Product Category: Active Pharmaceutical Ingredient (API)
-
Molecular Formula: C₁₃H₁₁N₃O₄
-
Molecular Weight: 273.24
Product Description
On January 8, 2013, the U.S. FDA approved Pomalyst (pomalidomide) for the treatment of multiple myeloma in patients whose disease has progressed after prior cancer therapies.
Multiple myeloma is a type of blood cancer that primarily affects older adults and originates from plasma cells in the bone marrow. According to the U.S. National Cancer Institute, approximately 21,700 people in the U.S. are diagnosed with multiple myeloma each year, and about 10,710 deaths are attributed to the disease.
Pomalyst is an oral medication that modulates the immune system to destroy cancer cells and inhibit their growth. It is indicated for patients who have received at least two prior therapies, including lenalidomide and bortezomib, and whose disease is refractory or has progressed within 60 days of the last treatment.
As an immunomodulatory drug (IMiD), pomalidomide is the third drug in its class, following thalidomide and lenalidomide. Treatment for multiple myeloma is often personalized, and Pomalyst offers a new option for patients with relapsed and refractory disease.
In July 2012, the FDA also approved Kyprolis (carfilzomib) for multiple myeloma. Like Kyprolis, Pomalyst was approved under the FDA’s accelerated approval program and granted orphan drug designation.
The safety and efficacy of Pomalyst were evaluated in a clinical trial involving 221 patients with relapsed or refractory multiple myeloma. The trial measured the objective response rate (ORR)—the percentage of patients whose cancer partially or completely responded to treatment. Patients were randomly assigned to receive Pomalyst alone or Pomalyst plus low-dose dexamethasone.
Results showed:
-
ORR of 7.4% for Pomalyst monotherapy. Median duration of response was not yet reached at the time of analysis.
-
ORR of 29.2% for Pomalyst + low-dose dexamethasone, with a median response duration of 7.4 months.
Pomalyst carries a boxed warning alerting patients and healthcare providers that it must not be used during pregnancy due to the risk of life-threatening birth defects and potential for blood clots.
Due to these risks, Pomalyst is distributed under a Risk Evaluation and Mitigation Strategy (REMS) program. Under REMS, physicians and patients must sign an agreement. Women of childbearing potential must undergo pregnancy testing and use contraception; male patients must also comply with contraceptive measures. Pharmacies must be REMS-certified and only dispense the drug to eligible patients.
Common side effects include:
-
Neutropenia (low white blood cell count)
-
Fatigue and weakness
-
Anemia (low red blood cell count)
-
Constipation or diarrhea
-
Thrombocytopenia (low platelet count)
-
Upper respiratory infections
-
Back pain
-
Fever
Keywords
19171-19-8; Pomalidomide; Pomalyst; Multiple Myeloma Treatment; Immunomodulatory Drug; Relapsed and Refractory Myeloma; FDA Orphan Drug; Oral Chemotherapy; IMiD; Anti-cancer API